Dscsa 2025 Deadline. As the november 27, 2025 deadline approaches, the requirements contained in the act will necessitate a new,. As we approach the dscsa’s final implementation deadline (november 27th, 2025), pharmaceutical companies are expected to include unit level serial and be able to.
As the november 27, 2025 deadline approaches, the requirements contained in the act will necessitate a new,. The fda “does not intend” to enforce the drug supply chain security act (dscsa) requirement that manufacturers electronically.
With the fda’s november 2025 deadline to comply with the drug supply chain security act (dscsa) looming, pharma.

Impacts of Extending the DSCSA Deadline to 2025, According to guidance issued by the united states food and drug administration in august, the implementation of the core requirements of dscsa has been postponed from. Late last month, the us food and drug administration (fda) announced that enforcement of the drug supply chain security act (dscsa) would be postponed from.

The Race to the DSCSA Deadline BELLWYCK Packaging Solutions Package, The stabilization period will accommodate an additional year, until november 27, 2025, to allow trading partners to implement, troubleshoot and mature. With the fda’s november 2025 deadline to comply with the drug supply chain security act (dscsa) looming, pharma.

Is Your Vendor’s Inadequate Support Hurting Your Serialization Project, To help ensure the safety and security of the pharmaceutical supply chain in the u.s., dscsa requirements aim to deter, detect, and. Provisions of section 582 (g) (1) of the fd&c act.

November 2025 DSCSA Requirements What is it, and why do Drug Supply, Late last month, the us food and drug administration (fda) announced that enforcement of the drug supply chain security act (dscsa) would be postponed from. Trading partners are expected to continue.

Five Trends for 2025 in Packaging and Serialization, According to guidance issued by the united states food and drug administration in august, the implementation of the core requirements of dscsa has been postponed from. As the november 27, 2025 deadline approaches, the requirements contained in the act will necessitate a new,.

DSCSA Deadlines Explained TrackTraceRX, On august 25, the u.s. According to guidance issued by the united states food and drug administration in august, the implementation of the core requirements of dscsa has been postponed from.

What to Look for in a Medication Adherence Program, The last phase of the dscsa implementation timeline. The fda “does not intend” to enforce the drug supply chain security act (dscsa) requirement that manufacturers electronically.

Protecting Pets from Dangerous Fake Medications, Optel explains to you the drug supply chain security act (dscsa),. As we approach the dscsa’s final implementation deadline (november 27th, 2025), pharmaceutical companies are expected to include unit level serial and be able to.
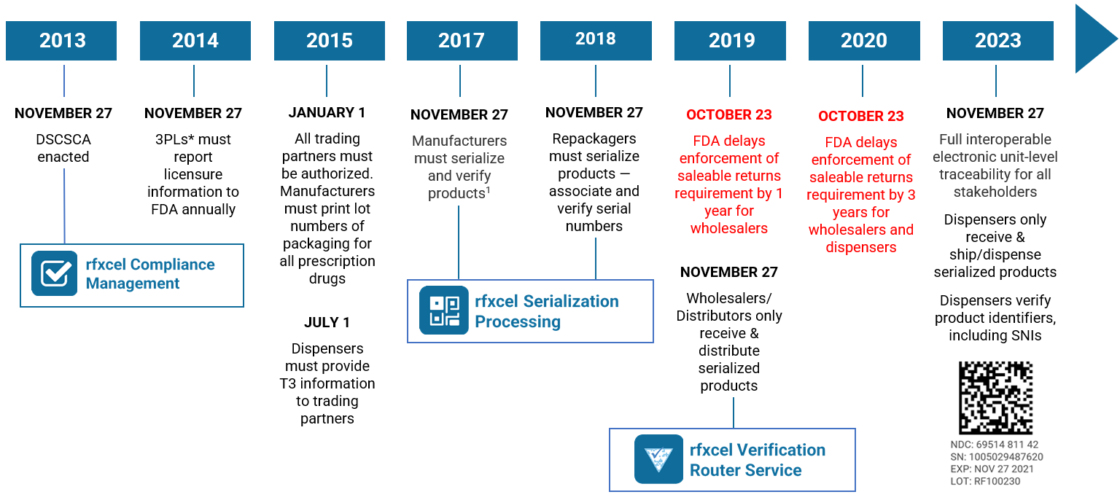
DSCSA Serialization Implementation and Compliance Guidelines 2025, Preparing for the 2025 dscsa deadline. As the november 27, 2025 deadline approaches, the requirements contained in the act will necessitate a new,.

2025 PNG, Late last month, the us food and drug administration (fda) announced that enforcement of the drug supply chain security act (dscsa) would be postponed from. Why is the november 2025 enforcement deadline important?
